Researchers at the University of Cambridge have identified a process called graphitization, which they theorize could produce molecules essential for building life such as proteins, phospholipids, and nucleotides on early Earth. This process, highlighted in a study published in the journal Life, suggests that high temperatures resulting from celestial influences and interactions with iron and water could streamline chemical environments, making them favorable for the formation of components necessary for life.
Researchers at the University of Cambridge suggest that molecules essential to the evolution of life may have arisen from a process called graphitization. If this is confirmed through laboratory experiments, it may enable us to simulate the conditions that likely gave rise to life.
How did the chemicals needed for life get there?
How the seemingly fortuitous conditions for life arose in nature has long been debated, with many hypotheses reaching dead ends. However, researchers at the University of Cambridge have now modeled how these conditions occur, producing the components necessary for life in large quantities.
Life is governed by molecules called proteins, phospholipids, and nucleotides. Previous research suggests that beneficial nitrogen-containing molecules such as nitrile – Cyanoacetylene(HC3N) and Hydrogen cyanide(HCN) – and isonitrile – Isocyanide(HNC) and Methyl isocyanide(CH3NC) – Can be used to make these essential elements of life. So far, there's no obvious way to make all of these things in the same environment in large quantities.
In a recent study published in lifeThe group has now found that, through a process known as graphite, large quantities of these beneficial molecules can theoretically be synthesized. If the model can be verified experimentally, it would suggest that the process was a possible early Earth step on its journey toward life.
Why is this process more likely to occur than others?
The biggest problem with previous models is that a host of other products are created alongside nitrile. This creates a chaotic system that hinders the formation of life.
“A big part of life is simplicity,” said Dr. Paul Rimmer, assistant professor of experimental astrophysics at Cavendish Laboratory and co-author of the study. “It's the system.” We've figured out a way to eliminate some of the complexity by controlling what can happen in chemistry.
We do not expect life to be produced in a chaotic environment. So, what's amazing is how graphite itself cleans the environment, as the process produces exclusively these nitriles and isonitriles, with mostly inert side products.
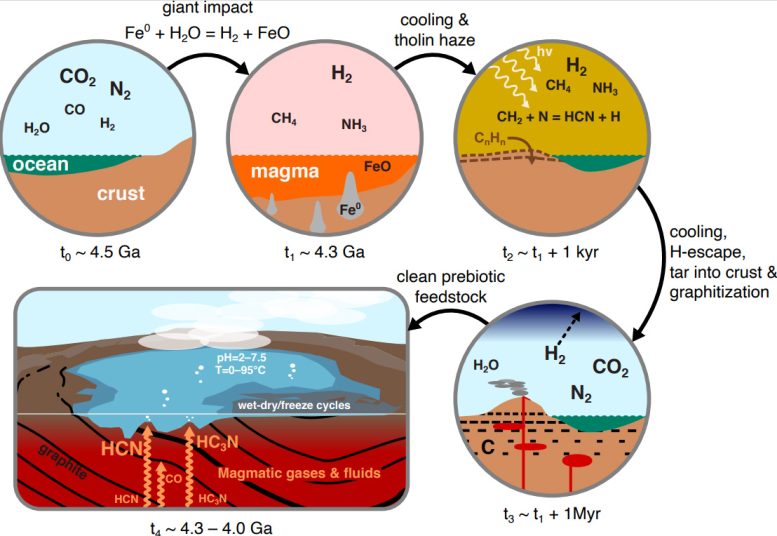
A schematic representation of the scenario we propose here for the production of clean, high-yield prebiotic raw materials. Events move clockwise from top left: First, Earth has a neutral atmosphere. This is reduced after a giant 4.3 G impact by oxidizing the collider's metallic core to produce a massive amount of hydrogen.2 Atmosphere with large amounts of methane and ammonia. This atmosphere cools rapidly (in less than one year), and photochemistry produces a tholin-rich fog that deposits complex, nitrogen-rich organic materials. These organic materials become gradually buried and drawn by interaction with magma. The skies become clear as H2 It gets lost in space and becomes neutral again. Finally, the molten gases react with the graphite and are rubbed to produce high amounts of clean HCN and HC.3N, isonitrile. Credit: Oliver Shortle
“At first, we thought this would ruin everything, but it actually makes everything so much better. It cleans up the chemistry,” Rimmer said.
This means that graphite could provide the simplicity that scientists are looking for, and the clean environment needed for life.
How does this process work?
The Hadean Era was the earliest period in Earth's history, when the Earth was very different from our modern Earth. Not surprisingly, collisions with debris, sometimes the size of planets, occurred. The study posits that when the early Earth collided with an object roughly the size of the Moon, about 4.3 billion years ago, the iron in it interacted with water on Earth.
Co-author Dr Oliver Shortle, Professor of Natural Philosophy at the Institute of Astronomy and Department of Earth Sciences at Cambridge, said: 'Something the size of the Moon struck the Earth early and would have deposited a large amount of iron and other metals.'
The reaction products of iron and water condense to form tar on the surface of the earth. The tar then reacts with the magma at a temperature of over 1500°C and the carbon in the tar turns into graphite – a very stable form of carbon – which is what we use in modern pencils!
Once the iron reacts with water, fog is formed that condenses and mixes with the Earth's crust. “When heated, what's left behind are the beneficial nitrogen-containing compounds,” Shortell said.
What evidence is there to support this idea?
Evidence supporting this theory comes in part from the presence of komatiite rocks. Komatite is a type of igneous rock that forms when very hot magma cools (>1500°C).
Komatite was originally found in South Africa. “The rocks date back to about 3.5 billion years ago,” Shortell said. “More importantly, we know that these rocks only form at extreme temperatures, around 1,700 degrees Celsius!” This means that the magma was already hot enough to heat the tar and form the useful nitrile.
With the link confirmed, the authors suggest that nitrogen-containing compounds will be synthesized via this method. Since we see komatite, we know that the temperature of magma on early Earth was sometimes over 1,500 degrees Celsius.
What then?
Now experiments must try to recreate these conditions in the laboratory, and study whether water, inevitably present in the system, eats up the nitrogen compounds, breaking them down.
“Although we don't know for sure that these molecules started life on Earth, we do know that the building blocks of life must have been made of molecules that survived in water,” Reimer said. “If future experiments show that nitrile breaks down, we will have to look for a different method.”
Reference: “Surface hydrothermal source of nitrile and isonitrile” by Paul P. Rimmer and Oliver Shortle, 10 April 2024, life.
doi: 10.3390/life14040498
The study was funded by a Cambridge Research Grant for Planetary Science and Life in the Universe.

“Explorer. Unapologetic entrepreneur. Alcohol fanatic. Certified writer. Wannabe tv evangelist. Twitter fanatic. Student. Web scholar. Travel buff.”