Japanese researchers at Yokohama National University have demonstrated a promising alternative to nickel-cobalt batteries for electric vehicles.
Their approach relies on using manganese in the anode to create a battery with high energy density, which is cost-effective and sustainable.
Electric vehicle manufacturers favor nickel-cobalt batteries because they offer higher energy density, meaning longer range in a smaller battery pack. However, both components are very expensive and relatively scarce, making them unsustainable options as electric vehicle use rises worldwide.
Lithium-ion (Li-ion) batteries are the preferred choice for rechargeable batteries in most electronic devices. However, their low energy density puts them at a disadvantage compared to electric vehicles. Research and development efforts to improve them have led to better lithium-ion battery options.
Experiments have also been conducted with manganese in anode materials besides lithium, such as LiMnO2. However, applications have been limited by the poor performance of the electrode. Researchers at Yokohama National University (YNU) in Japan have addressed this issue in their recent work.
Working on a single-pitch system
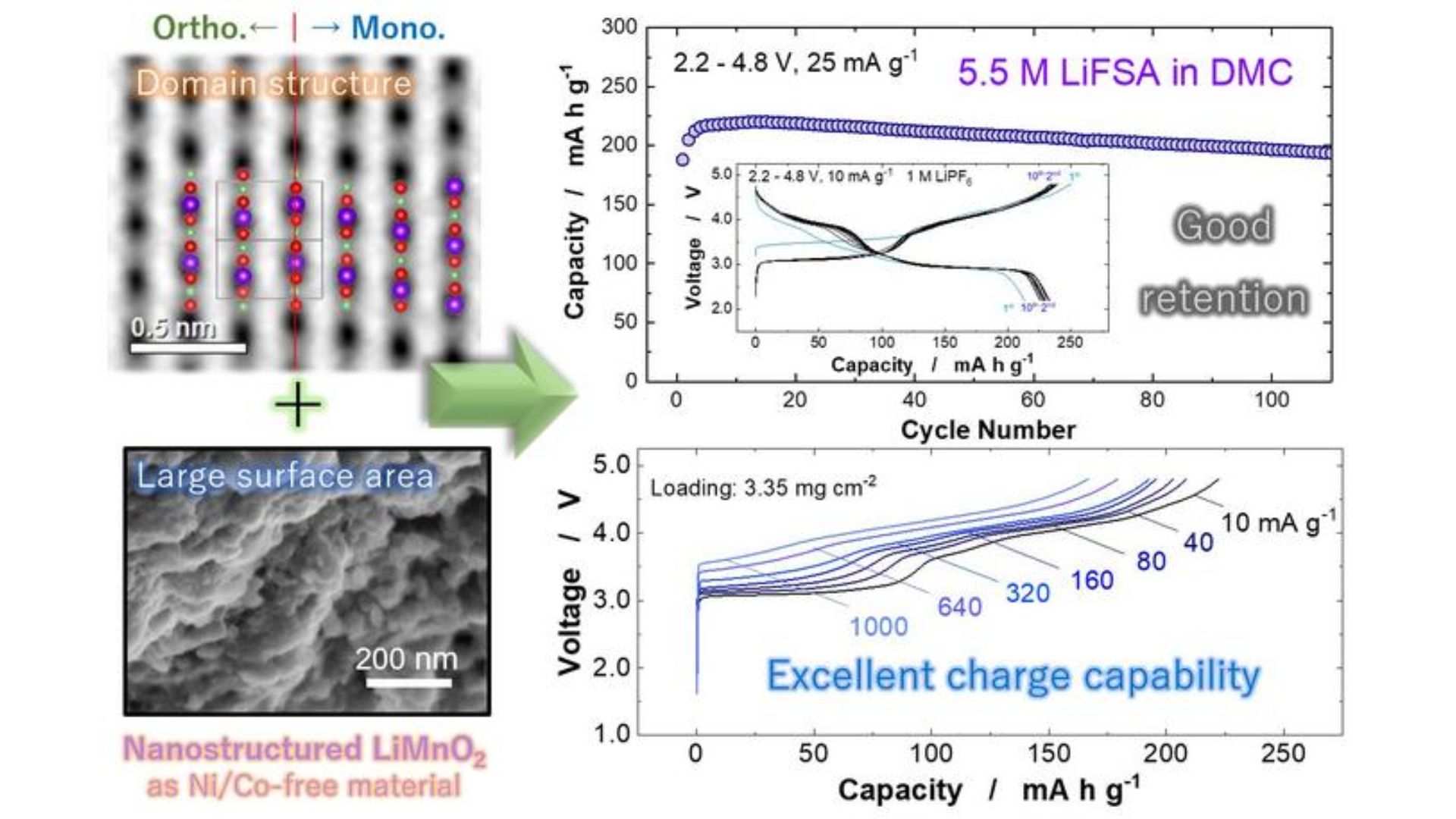
After extensively studying LiMnO2 in its various forms using X-ray diffraction, scanning electron microscopy, and electrochemical methods, researcher Naoki Yabuchi and his team at Yangon University found that the monoclinic layered domain activates the structural transformation of LiMnO2 into a spinel-like phase. The monoclinic system is a kind of collective symmetry of the solid crystal structure.
LiMnO2 improves the performance of the electrode material by facilitating phase transition. Without phase transition, the performance of the LiMnO2 electrode is less than optimal.
“From this discovery, nanoscale LiMnO2 with monoclinic layered domain structures and high surface area were directly synthesized using a simple solid-state reaction,” Yabuchi said in press release.
The reaction does not involve any intermediate steps and can be manufactured directly from two components using the calcination process.
Performance improvements with Mn
Post-assembly testing revealed that the LiMnO2 electrode battery achieved an energy density of 820 Wh/kg-1 compared to 750 Wh/kg-1 obtained with a nickel-based battery. Only lithium-based batteries had a lower energy density of 500 Wh/kg-1.
The researchers said Interesting engineering In an email, manganese, when used in other forms, typically exhibits half the energy density.
A previous study using manganese showed that the voltage drop in batteries decreased over time, which reduced the performance of the electronic device. However, the researchers did not observe such results with the LiMnO2 electrode.
Manganese degradation can still occur, either due to phase changes or interaction with an acidic solution. The researchers plan to address this issue using a high-concentration electrolyte solution and lithium phosphate coating, the press release added.
The researchers are confident that their work has contributed to the development of a new product that is competitive with existing options, sustainable to produce, and environmentally friendly in the long term. They are looking to commercialize their technology and use it in the electric vehicle industry.
“We found a very cheap methodology, and this is the important finding in our study,” the research team added in their email to IE.
About the Editor
Amia Baliga Amiya is a science writer based in Hyderabad, India. A molecular biologist at heart, he gave up his micropipette to write about science during the pandemic and doesn’t want to go back. He loves writing about genetics, microbes, technology, and public policy.

“Web specialist. Lifelong zombie maven. Coffee ninja. Hipster-friendly analyst.”