A Northwestern University study presents a cost-effective catalyst made from molybdenum and table sugar that converts carbon dioxide into carbon monoxide, providing a viable way to convert captured carbon into useful products such as fuel precursors.
The new catalyst may provide a potential solution for using captured carbon.
A new catalyst made from an inexpensive and abundant metal, common table sugar, has the ability to destroy carbon dioxide (CO2).2) Gas.
In a new study conducted by Northwestern University, the catalyst successfully converted carbon dioxide2 to carbon monoxide (CO), an important building block for the production of a variety of beneficial chemicals. When the reaction occurs in the presence of hydrogen, for example, CO2 Hydrogen is converted into synthetic gas (or syngas), a valuable material for producing fuel that can replace gasoline.
With recent advances in carbon capture technologies, post-combustion carbon capture has become a reasonable option to help address the global climate change crisis. But how to deal with captured carbon remains an open question. The new catalyst potentially provides a solution to eliminating the powerful greenhouse gas by converting it into a more valuable product.
The study will be published in the May 3 issue of the magazine Sciences.
“Even if we stop emitting carbon dioxide2 Now, our atmosphere will still contain an excess of carbon dioxide2 “It is the result of industrial activities from past centuries,” said Milad Khoshoui of Northwestern University, who co-led the study. “There is no single solution to this problem. We need to reduce carbon dioxide2 emissions And Find new ways to reduce carbon dioxide2 Concentration already present in the atmosphere. We must take advantage of all possible solutions.”
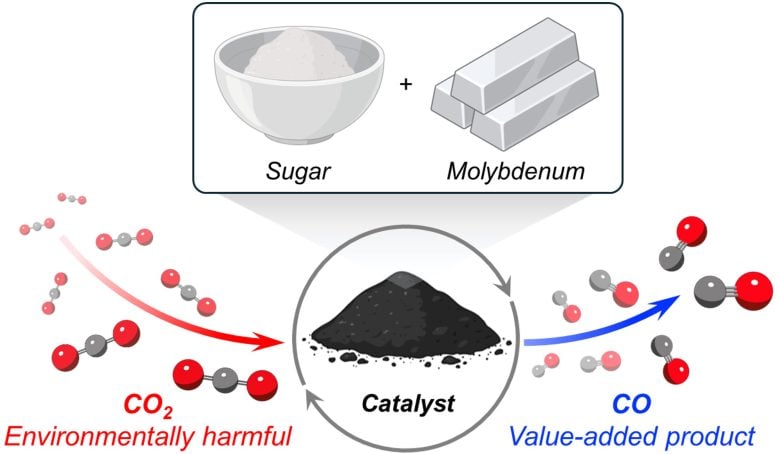
This diagram shows the complete process of creating a catalyst and using it to convert carbon dioxide. Credit: Milad Khoshoui
“We are not the first research group to convert carbon dioxide2 “On to another product,” Omar K. said. Farha, of Northwestern University, is the study’s senior author. “However, for the process to be truly practical, it requires a catalyst that meets several critical criteria: affordability, stability, ease of production, and scalability. Balancing these four elements is key. Fortunately, our material excels at meeting These requirements.
An expert in carbon capture technologies, Farha is the Charles E. and Emma H. Morrison Professor of Chemistry in the Weinberg College of Arts and Sciences at Northwestern. After starting this work with a Ph.D. Khoshwai is a candidate at the University of Calgary in Canada, and is now a postdoctoral fellow in Farha’s lab.
Solutions from the store
The secret behind the new catalyst is molybdenum carbide, an extremely hard ceramic material. Unlike many other catalysts that require expensive metals, such as platinum or palladium, molybdenum is an inexpensive, non-precious metal that is abundant in the Earth.
To convert molybdenum into molybdenum carbide, scientists needed a source of carbon. They discovered a cheap option in an unexpected place: the pantry. Surprisingly, sugar – the white, granular kind found in almost every household – served as a convenient and inexpensive source of carbon atoms.
“Every day I was trying to manufacture these materials, I was bringing sugar from my home to the laboratory,” Khoshoui said. “When compared to other classes of materials commonly used in catalysts, our products are incredibly inexpensive.”
Successfully and stable selectivity
When testing the catalyst, Farha, Khoshoui and their collaborators were impressed by its success. Operates at ambient pressures and high temperatures (300-600 degrees). Celsius), the converted catalyst CO2 to carbon dioxide with 100% selectivity.
High selectivity means that the catalyst operates only on carbon dioxide2 Without damaging the surrounding materials. In other words, industry can apply the catalyst to large amounts of captured gases and selectively target only carbon dioxide.2. The catalyst also remained stable over time, meaning it remained active and did not decompose.
“In chemistry, it is not uncommon for a catalyst to lose its selectivity after a few hours,” Farha said. “But after 500 hours in harsh conditions, its selectivity did not change.”
This is especially notable because CO2 It is a stable and stubborn molecule.
“CO conversion2 “It’s not easy,” Khoshuai said. “Ko2 It is a chemically stable molecule, and we had to overcome this stability, which requires a lot of energy.
The tandem approach to carbon cleaning
Developing materials needed to capture carbon is the main focus of Farha Lab. His group is developing metal-organic frameworks (Metal organic frameworks), a class of highly porous, nano-sized materials that Farha likens to “sophisticated, programmable bath sponges.” Farha is exploring MOFs for diverse applications, including carbon dioxide capture2 Directly from the air.
Now, Farha says the MOF and the new catalyst could work together to play a role in carbon capture and sequestration.
“At some point, we could use MOFs to capture carbon dioxide, followed by a catalyst to convert it into something more useful,” Farha suggested. “A tandem system that uses two different materials for two sequential steps could be the way forward.”
“This may help us answer the question: What do we do with captured carbon dioxide?2“Khushui added. “Right now, the plan is to isolate it underground. But underground reservoirs must meet several requirements in order to store carbon dioxide safely and permanently.”2. We wanted to design a more universal solution that could be used anywhere while adding economic value.
Reference: “Active and stable cubic molybdenum carbide catalyst for high-temperature water-gas reversible transformation reaction” by Milad Ahmadi Khoshui, Shijun Wang, Gerardo Vitale, Philip Formalek, Kent O. Kerlikovalli, Randall Q. Senor, Pedro Pereira-Almao, and Omar K. Farha, May 2, 2024, Sciences.
doi: 10.1126/science.adl1260
This study was supported by the US Department of Energy, the National Science Foundation, and the Natural Sciences and Engineering Research Council of Canada.

“Explorer. Unapologetic entrepreneur. Alcohol fanatic. Certified writer. Wannabe tv evangelist. Twitter fanatic. Student. Web scholar. Travel buff.”