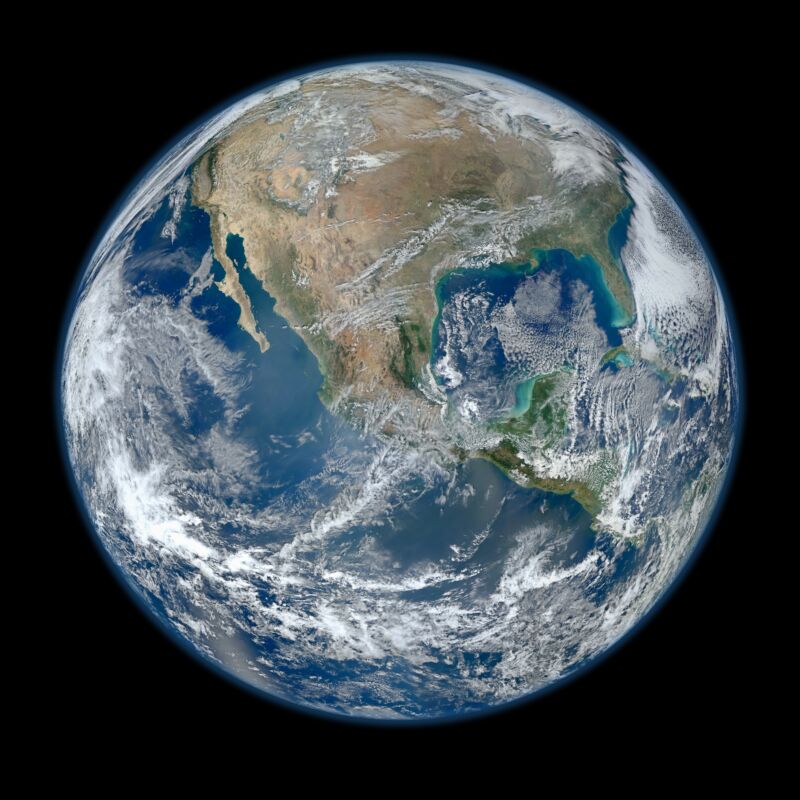
Water made Earth what it is – a planet known for its blue oceans. Water forms the Earth through erosion and is essential to the Earth’s ability to support life. But we have a hard time understanding how Earth ended up with all that water, since the building blocks that created it likely dried up, and the collisions that turned these building blocks into a planet should have pushed any surface water out into space. .
Various means of delivering water to the Earth after its formation have been proposed. But a new study takes information gleaned from examining exoplanets and applies it to Earth. The results indicate that the chemical reactions that would have taken place during Earth’s formation would have produced enough water to fill the world’s oceans. And as a side benefit, the model explains the somewhat strange density of Earth’s core.
waterproof
Earth seems to have been created primarily from materials in the inner solar system. Not only was that material in the right place, but the material in asteroids in the region provided a good match in terms of their elemental and isotopic compositions. But this material is also very dry. This is no surprise. Temperatures in this region would have prevented the water from condensing into a solid, as it can exist in the solar system, beyond a point known as the “ice line” of water.
Any water in space would have been lost, as it is believed that the planet-building process occurred by collisions between small bodies, with larger bodies gradually growing larger as smaller bodies continued to collide with them. Much of the water in these objects would evaporate and possibly be lost to space.
But three researchers (Edward Young, Anat Shahar, and Hilke Schlichting) focused on an additional factor that could have been present during the formation of the solar system: hydrogen. Hydrogen is thought to be present in large quantities during the early period of planet formation, but is then pushed out by the radiation released once the central star ignites. In our solar system, some of it was captured by the outer planets before it was lost. But our inner planets seem to have formed with little or no element early in their history.
But a look at the exoplanets suggests that this is not an inevitable fate. We’ve found several super-rocky planets that also seem to lack hydrogen-rich atmospheres. But there is a gap about twice the Earth’s radius where we see a lot of young Neptune, which seems to have retained a thick, probably hydrogen-rich atmosphere. This has led to the suggestion that all rocky planets begin in a hydrogen-rich environment and form their first atmospheres from that. But below a certain size, this hydrogen is lost later in their history. Any atmospheres present on these planets are likely the result of secondary formation.
Taking that to its logical conclusion, Earth may have begun with a hydrogen-rich atmosphere as well. Therefore, the researchers involved in the new study decided to look at what the consequences of this scenario could be.
Planetary chemistry
To explore this idea, the researchers essentially modeled a giant chemical reactor filled with most of the components of the early Earth and expanded to the size of a large Earth precursor (half the size of the current Earth). This includes things like oxides of iron, sodium, various silicates, carbon dioxide, methane, oxygen, and more. All of this was placed under a hydrogen-rich atmosphere and heated to reflect oceans of magma from the repeated collisions that occurred during planet formation.
This period likely lasted tens of millions of years, in part because hydrogen atmospheres tend to retain heat very well (they can act as a greenhouse gas). This gives the chemical reactions taking place – 18 of which the researchers tracked – time to reach equilibrium and enough time for the different materials in the planet’s interior to split up based on density.
One of the things that happens is that many elements are incorporated into the iron core, including oxygen, silicon, and hydrogen. Since all of these are less dense than iron, this has the effect of making the core less dense than it would be if it were pure iron – which is true of actual Earth.
In some reactions, the fusing of hydrogen involves the displacement of oxygen, and the byproduct of these reactions is water. Under the conditions explored here, the reactions produce the same volume as found in Earth’s current oceans. “Even if the rocks in the inner solar system are completely dry,” the researchers wrote, the reactions between H.2 The atmosphere and magma oceans will generate abundant amounts of H2O. Other sources of H2O is possible, but not required.
modeling limits
On the plus side, the simulation works with a wide range of temperatures — all it takes is enough heat to keep the planet melting while the processes described here reach equilibrium. It also works for different sizes of precursors, but it fails if the precursor is too small. This corresponds to the extreme dryness of Mars and Mercury. The primary variable ends with the amount of water being produced; If more hydrogen ended up in the core, it could easily create a water world three times the size of today’s oceans.
While the model is robust to many changes in initial conditions, it is limited by not being a complete picture of the chemistry of the early Earth. It is worth noting that sulfur and nitrogen have played major roles in Earth’s chemistry.
But the big gap in the model is what happens after water forms. Because there is an ocean of magma, it will end up in the atmosphere, where it can be split off by solar radiation and lost if the hydrogen in the solar system does indeed dissipate. The same is true of any aftereffects that warmed the planet, such as the giant impact that shaped the moon. If there is still enough hydrogen, this is not a problem because the water can fix it. The researchers cite research showing that a water-rich atmosphere could survive even a massive impact. Finally, you can imagine conditions in which an initial excess of water was produced, but enough was lost through these processes to leave the Earth in its present state.
So, while producing water requires no fine-tuning of conditions, retaining it may.
But the implications for worlds outside our own seem a bit bigger. These results indicate that a wide range of initial conditions must have produced water during the formation of rocky planets. Therefore, when we think of planets in exosystems, it might be more questionable to ask if they experienced conditions that would have caused them to lose water than to ask if they could have had any in the first place.
Nature, 2023. DOI: 10.1038 / s41586-023-05823-0 (about DOIs).

“Explorer. Unapologetic entrepreneur. Alcohol fanatic. Certified writer. Wannabe tv evangelist. Twitter fanatic. Student. Web scholar. Travel buff.”


